Separating Sulphur
David Jackson, Merichem Process Technologies, USA, explores H2S removal with liquid redox processes.
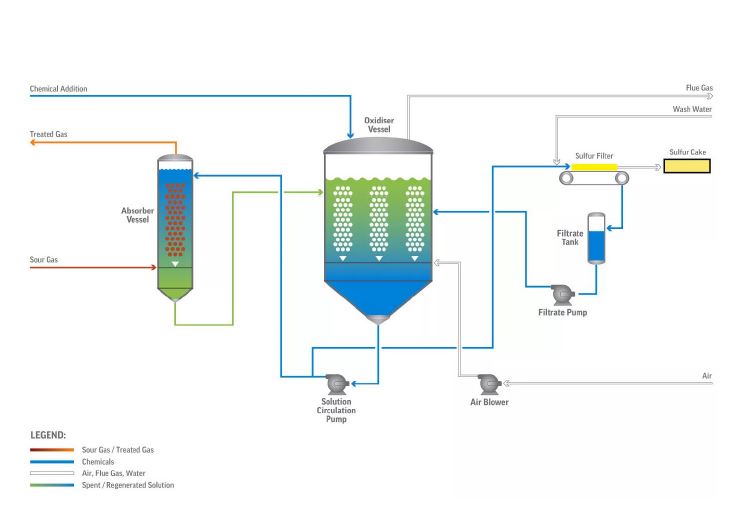
Liquid redox is a process used to remove hydrogen sulfide (H2S) from sour natural gas and other gas streams by converting it to solid elemental sulfur for sale or disposal. It is based on a reduction-oxidation reaction carried out in an aqueous solution and catalysed by iron which is held in solution by organic chelating agents. The spent solution is then circulated to an oxidiser to be regenerated with air and reused. This process forms solid sulfur particles that can be easily filtered from the solution. As shown in Figure 1, the typical applications are streams where the total amount of sulfur removed is greater than 0.5 tpd and less than 20 tpd. Liquid redox processes are not usually economical for streams that contain very small amounts of sulfur, where the total amount is less than several hundred pounds per day.
The niche for liquid redox processes can be found between two extremes – sour gas streams with high concentrations of H2S and a constant throughput near the design point, here the Claus process is utilised, and those with very dilute H2S concentrations, where non-regenerable scavengers are applied in towers or by direct injection. Liquid redox can process virtually any kind of gas stream and, as a result, the technology has been applied in a wide variety of applications to achieve 99.9%+ H2S removal in a single stage.
More information here: PDF
